Dr. Micah T. Long is an anesthesia-intensivist who views himself as a “clinician-first.” Accordingly, his lab tackles a diverse grouping of late-stage translational clinical research that has impacted his patients directly at the bedside.
Currently, Dr. Long is working on the following research efforts:
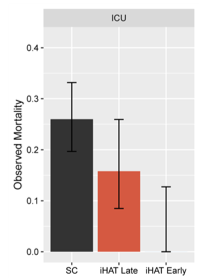
- In-Hospital Cardiac Arrest. Dr. Long is the PI for a retrospective multicenter assessment of calcium-use for in-hospital cardiac arrest (IHCA) – the only trial in this area that will be time-matched to best understand the drugs utility (or risk) in IHCA management. I am also assessing the impact of a dedicated nursing code team on CPR quality.
- Emergency Tracheal Intubation. Dr. Long was the site co-PI for the multicenter Preoxi trial assessing the approach to preoxygenation for urgent/emergent intubation, and he is the UW site-lead for the Pragmatic Critical Care Research Group (PCCRG) for upcoming studies. He has developed an airway registry at UW to better assess airway outcomes and contributors to those outcomes, that he hopes to deploy within the Midwest Anesthesia Consortium as descriptive and interventional trials involving intubation in the critically ill. Dr. Long is also involved in post-intubation management including practices surrounding the reversal of rocuronium in these patients (the Roc & Roll retrospective trial), and in airway-checklists / cognitive aids, including a simulation-based trial entitled SimCheck assessing checklist-use in trainees.
- Vitamin C and Ischemia/reperfusion injury in organ transplantation. Dr. Long published the only time-matched trial evaluating vitamin C in septic shock, which showed an extremely time-sensitive impact of the therapy (https://pubmed.ncbi.nlm.nih.gov/32484528/). He has routinely focused on resuscitative approaches to drugs and patient management (https://pubmed.ncbi.nlm.nih.gov/35644899/). He was the PI for a funded trial assessing vitamin C in lung transplantation (FDA-IND cleared; clinicaltrials.gov NCT04505878), and is the co-PI for a similar study in liver transplantation – the PARTi Trial (Parenteral Ascorbate Repletion in Transplantation; FDA-IND cleared; Clinicaltrials.gov NCT04756063).
- Dr. Long is an expert in perioperative and critical care glycemic monitoring and glucose control (e.g. https://pubmed.ncbi.nlm.nih.gov/31800411/, https://pubmed.ncbi.nlm.nih.gov/35916913/ and https://pubmed.ncbi.nlm.nih.gov/30198923/). He is also an expert on the diagnosis and management of non-hepatic hyperammonemia in the critically ill (https://pubmed.ncbi.nlm.nih.gov/35447602/ and https://pubmed.ncbi.nlm.nih.gov/37938118/).
Publications
Dr. Long’s Pubmed-indexed publications can be found here:
